Project B6
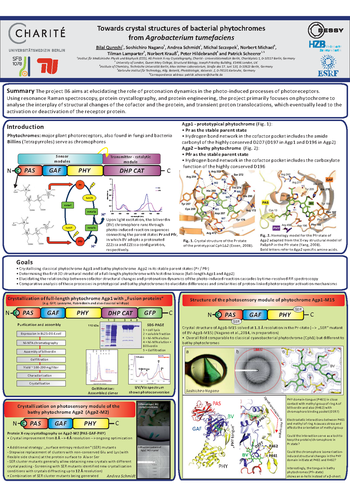
Award-Winning Poster by Dr. Bilal Qureshi (project B6 / AG Scheerer): Towards crystal structures of bacterial phytochromes from Agrobacterium tumefaciens (Bad Schandau, 2014)
Proton-coupled conformational changes in photoreceptors
Principal Investigators: Prof. Dr. Peter Hildebrandt (TU), Dr. Patrick Scheerer (Charité)
By combining structural biology methods, protein engineering, and vibrational spectroscopy, we aim at determining the structural basis and dynamics of the proton-dependent reaction steps in phytochromes. Specific objectives are to analyze (i) the differences in the protonation-dependent reaction mechanism along the Pr/Pfr and Pfr/Pr transformations of prototypical and bathy bacteriophytochromes, (ii) different proton-dependent mechanisms of photosensor activation in prokaryotic and eukaryotic phytochromes, and (iii) cause-effect relationships between movements of chromophore, protons, and protein building blocks.
Publications
2021 - 2024
Batebi, H., Perez-Hernandez, G., Rahman, S.N., Lan, B., Kamprad, A., Shi, M., Speck, D., Tiemann, J.K.S., Guixa-Gonzalez, R., Reinhardt, F., Stadler, P.F., Papasergi-Scott, M.M., Skiniotis, G., Scheerer, P., Kobilka, B.K., Mathiesen, J.M., Liu, X. and Hildebrand, P.W. (2024). Mechanistic insights into G-protein coupling with an agonist-bound G-protein-coupled receptor. Nat Struct Mol Biol. doi: 10.1038/s41594-024-01334-2.
Blain-Hartung, M., Sass, G.J. von., Plaickner, J., Hoang, O.T., Katz, S., Mroginski, M.A.¸ Esser, N., Budisa, N., Forest, K.T., and Hildebrandt, P. (2024). On the Role of a Conserved Tryptophan in the Chromophore Pocket of Cyanobacteriochrome. J Mol Biol. doi: 10.1016/j.jmb.2023.168227.
Broser, M., Andruniow, T., Kraskov, A., Palombo, R., Katz, S., Kloz, M., Dostál, J., Bernardo, C., Kennis, J., Hegemann, P., Olivucci M., and Hildebrandt, P. (2023). Experimental assessment of the electronic and geometrical structure of a near-infrared absorbing and highly fluorescent microbial rhodopsin. J Phys Chem Lett. doi: 10.1021/acs.jpclett.3c02167.
Elkurdi, A., Guigas, G., Hourani-Alsharafat, L., Scheerer, P., Nienhaus, G. U., Krauß, N., and Lamparter, T. (2023). Time-resolved fluorescence anisotropy with Atto 488-labeled phytochrome Agp1 from Agrobacterium fabrum. Photochem Photobiol. doi: 10.1111/php.13851.
Gonzalez, B.D., Forbrig, E., Yao, G., Kielb, P., Mroginski, M.A., Hildebrandt, P. and Kozuch, J. (2024). Cation Dependence of Enniatin B/Membrane-Interactions Assessed Using Surface-Enhanced Infrared Absorption (SEIRA) Spectroscopy. Chempluschem, 89, 8: e202400159. doi: 10.1002/cplu.202400159.
Hildebrandt, P. (2023). Vibrational spectroscopy of phytochromes. Biomolecules 13: 1007. doi: 10.3390/biom13061007.
Kaeser, G., Krauss, N., Roughan, C., Sauthof, L., Scheerer, P. and Lamparter, T. (2023). Phytochrome-Interacting Proteins. Biomolecules, 14, 1. doi: 10.3390/biom14010009.
Kraskov, A., Buhrke, D., Scheerer, P., Shaef, I., Sanchez, J.C., Carrillo, M., Noda, M., Feliz, D., Stojković, E.A., and Hildebrandt, P. (2021). On the Role of the Conserved Histidine at the Chromophore Isomerization Site in Phytochromes. J Phys Chem B, 125, 50: 13696-13709. doi: 10.1021/acs.jpcb.1c08245.
Kraskov, A., von Sass, J., Nguyen, A.D., Hoang, T.O., Buhrke, D., Katz, S., Michael, N., Kozuch, J., Zebger, I., Siebert, F., Scheerer, P., Mroginski, M.A., Budisa, N., and Hildebrandt P. (2021). Local Electric Field Changes during the Photoconversion of the Bathy Phytochrome Agp2. Biochemistry, 60, 40: 2967-2977. doi: 10.1021/acs.biochem.1c00426.
Kruse, F., Nguyen, A.D., Dragelj, J., Heberle, J., Hildebrandt, P., Mroginski, M.A., and Weidinger, I.M. (2021). A Resonance Raman Marker Band Characterizes the Slow and Fast Form of Cytochrome c Oxidase. J Am Chem Soc, 143: 2769-2776. doi: 10.1021/jacs.0c10767.
Lamparter, T., Xue, P., Elkurdi, A., Kaeser, G., Sauthof, L., Scheerer, P., and Krauß, N. (2021). Phytochromes in Agrobacterium fabrum. Front Plant Sci, 12. doi: 10.3389/fpls.2021.642801.
Lopez, M.F., Dahl, M., Escobar, F.V., Bonomi, H.R., Kraskov, A., Michael, N., Mroginski, M.A., Scheerer, P. and Hildebrandt, P. (2022). Photoinduced reaction mechanisms in prototypical and bathy phytochromes. Phys Chem Chem Phys, 24, 19: 11967-11978. doi: 10.1039/d2cp00020b.
Merga, G., Lopez, M.F., Fischer, P., Piwowarski, P., Nogacz, Ż., Kraskov, A., Buhrke, D., Escobar, F.V., Michael, N., Siebert, F., Scheerer, P., Bartl, F., and Hildebrandt, P. (2021). Light- and temperature-dependent dynamics of chromophore and protein structural changes in bathy phytochrome Agp2. Phys Chem Chem Phys, 23, 33, 18197-18205. doi: 10.1039/d1cp02494a.
Nguyen, A.D., Michael, N., Sauthof, L., von Sass, J., Hoang, O.T., Schmidt, A., La Greca, M., Schlesinger, R., Budisa, N., Scheerer, P., Mroginski, M.A., Kraskov, A. and Hildebrandt, P. (2024). Hydrogen Bonding and Noncovalent Electric Field Effects in the Photoconversion of a Phytochrome. J Phys Chem B. doi: 10.1021/acs.jpcb.4c06419.
Sass, G.J. von., Blain-Hartung, M., Baumann, T., Forest, K., Hildebrandt, P., Budisa, N. (2023). The repurposing of archaeal tyrosyl-tRNA synthetase in orthogonal translation with 5-cyanotryptophan as an infrared probe for local structural information, electrostatics, and hydrogen bonding. Prot. Sci. 32: e4705. doi: 10.1002/pro.4705.
Schmidt, A., Kalms, J., Lorent, C., Katz, S., Frielingsdorf, S., Evans, R. M., Fritsch, J., Siebert, E., Teutloff, C., Armstrong, F. A., Zebger, I., Lenz, O., and Scheerer, P. (2023). Stepwise conversion of the Cys6[4Fe-3S] to a Cys4[4Fe-4S] cluster and its impact on the oxygen tolerance of [NiFe]-hydrogenase. Chem Sci, 14, 40: 11105–11120. doi: 10.1039/d3sc03739h.
Silapetere, A., Hwang, S., Hontani, Y., Fernandez Lahore, R.G., Balke, J., Escobar, F.V., Tros, M., Konold, P.E., Matis, R., Croce, R., Walla, P.J., Hildebrandt, P., Alexiev, U., Kennis, J.T.M., Sun, H., Utesch, T. and Hegemann, P. (2022). QuasAr Odyssey: the origin of fluorescence and its voltage sensitivity in microbial rhodopsins. Nat Commun, 13, 1: 5501. doi: 10.1038/s41467-022-33084-4.
Smirnova, J., Loerke, J., Kleinau, G., Schmidt, A., Bürger, J., Meyer, E.H., Mielke, T., Scheerer, P., Bock, R., Spahn, C.M.T. and Zoschke, R. (2023). Structure of the actively translating plant 80S ribosome at 2.2 Å resolution. Nat Plants, 9, 6: 987-1000. doi: 10.1038/s41477-023-01407-y.
Vogt, A., Paulat, R., Parthier, D., Just, V., Szczepek, M., Scheerer, P., Xu, Q., Moglich, A., Schmitz, D., Rost, B.R. and Wenger, N. (2024). Simultaneous spectral illumination of microplates for high-throughput optogenetics and photobiology. Biol Chem, doi: 10.1515/hsz-2023-0205
Xue, P., Bai, Y., Rottwinkel, G., Averbukh, E., Ma, Y., Roeder, T., Scheerer, P., Krauß, N. and Lamparter, T. (2021). Phytochrome mediated responses in Agrobacterium fabrum: Growth, motility and plant infection. Curr Microbiol, 78, 2708-2719. doi: 10.1007/s00284-021-02526-5.
Yang, Y., Stensitzki, T., Sauthof, L., Schmidt, A., Piwowarski, P., Velazquez Escobar, F., Michael, N., Nguyen, A.D., Szczepek, M., Brünig, F. N., Netz, R.R., Mroginski, M.A., Adam, S., Bartl, F.J., Schapiro, I., Hildebrandt, P., Scheerer, P., and Heyne, K. (2022). Ultrafast proton-coupled isomerization in the phototransformation of phytochrome. Nat Chem. doi: 10.1038/s41557-022-00944-x.
2017 - 2020
Buhrke. D., Battocchio, G., Wilkening, S., Blain-Hartung, M., Baumann, T., Schmitt, F. J., Friedrich, F., Mroginski, M. A., Hildebrandt, P. (2020) Red, orange, green: Light- and Temperature-dependent Color Tuning in a Cyanobacteriochrome. Biochemistry 59, 509-519, doi: 10.1021/acs.biochem.9b00931.
Buhrke, D., Gourinchas, G., Müller, M., Michael, N., Hildebrandt, P., Winkler, A. (2020). Distinct chromophore-protein environments enable asymmetric activation of a bacteriophytochrome activated diguanylate cyclase. Journal of Biological Chemistry 295, 539-551. doi: 10.1074/jbc.RA119.011915.
Buhrke, D., Hildebrandt, P. (2020). Probing Structure and Reaction Dynamics of Proteins Using Time-Resolved Resonance Raman Spectroscopy. Chemical Reviews 120, 3577-3630. doi: 10.1021/acs.chemrev.9b00429.
Buhrke, D., Tavraz, N. N., Shcherbakova, D. M., Sauthof, L., Moldenhauer, M., Vélazquez Escobar, F., Verkhusha, V. V., Hildebrandt, P., Friedrich, T. (2019). Chromophore binding to two cysteines increases quantum yield of near-infrared fluorescent proteins. Scientific Reports 9: 1866. doi: 10.1038/s41598-018-38433-2.
Buhrke, D., Kuhlmann, U., Michael, N., and Hildebrandt, P. (2018). The Photoconversion of Phytochrome Includes an Unproductive Shunt Reaction Pathway. ChemPhysChem 19, 566-570; doi: 10.1002/cphc.201701311
Fernandez Lopez, M., Nguyen A. D., Velazquez Escobar, F., González, R., Michael, N., Nogacz, Ż., Piwowarski, P., Bartl, F., Siebert, F., Heise, I., Scheerer, P., Gärtner, W., Mroginski, M. A., Hildebrandt, P. (2019). Role of the Propionic Side Chains for the Photoconversion of Bacterial Phytochromes. Biochemistry 58, 3504-3519. doi: 10.1021/acs.biochem.9b00526
Fudim, R., Szczepek, M., Vierock, J., Vogt, A., Schmidt, A., Kleinau, G., Fischer, P., Bartl, F., Scheerer, P., Hegemann, P. (2019). Design of a light-gated proton channel based on the crystal structure of Coccomyxa rhodopsin. Science Signaling 12, 573. doi: 10.1126/scisignal.aav4203
Kacprzak, S., Njimona, I., Renz, A., Feng, J., Reijerse, E., Lubitz, W., Krauss, N., Scheerer, P., Nagano, S., Lamparter, T., and Weber, S. (2017). Inter-subunit distances in full-length, dimeric, bacterial phytochrome Agp1, as measured by PELDOR between different spin label positions, remain unchanged upon photoconversion. Journal of Biological Chemistry 292, 7598-7606; doi: 10.1074/jbc.m116.761882
Kielb, P., Utesch, T., Kozuch, J., Jeoung, J.-H., Dobbek, H., Mroginski, M.A., Hildebrandt, P., and Weidinger, I. (2017). Switchable Redox Chemistry of the Hexameric Tyrosine-Coordinated Heme Protein. Journal of Physical Chemistry B 121, 3955-3964; doi: 10.1021/acs.jpcb.7b01286
Kraskow, A., Nguyen, D. A., Buhrke, D., Velasquez Escobar, F., Fernandez Lopez, M., Michael, N., Sauthof, L., Schmidt, A., Piwowarski, P., Yang, Y., Stensitzki, T., Adam, S., Bartl, F., Schapiro, I., Heyne, K., Siebert, F., Scheerer, P., Mroginski, M. A., Hildebrandt, P. (2020) Intramolecular Proton Transfer Controls Protein Structural Changes in Phytochrome. Biochemistry 59, 1023-1037; doi: 10.1021/acs.biochem.0c00053.
Lamparter, T., Krauß, N., and Scheerer, P. (2017). Phytochromes from Agrobacterium fabrum. Photochemistry and Photobiology 93, 642-655; doi: 10.1111/php.12761
Luck, M., Velázquez Escobar, F., Glass, K., Sabotke, M. I., Hagedorn, R., Corellou, F., Siebert, F., Hildebrandt, P., Hegemann, P. (2019). Photoreactions of the histidine kinase rhodopsin Ot-HKR from the marine picoalga Ostreococcus tauri. Biochemistry 58, 1878-1891. doi: 10.1021/acs.biochem.8b01200.
Nagano, S., Guan, K., Shenkutie, S., Feiler, C., Weiss, M. S., Kraskov, A., Buhrke, D., Hildebrandt, P., Hughes, J. (2020). Structural insights into photoactivation and signalling in plant phytochromes. Nature Plants 6, 581-588; doi: 10.1038/s41477-020-0638-y
Oberthuer, D., Knoška, J., Wiedorn, M.O., Beyerlein, K.R., Bushnell, D.A-, Kovaleva, E.G., Heymann, M., Gumprecht, L., Kirian, R.A., Barty, A., Mariani, V., Tolstikova, A., Adriano, L., Awel, S., Barthelmess, M., Dörner, K., Xavier, P.L., Yefanov, O., James, D.R., Nelson, G., Wang, D., Calvey, G., Chen, Y., Schmidt, A., Szczepek, M., Frielingsdorf, S., Lenz, O., Snell, E., Robinson, P.J., Šarler, B., Belšak, G., Maček, M., Wilde, F., Aquila, A., Boutet, S., Liang, M., Hunter, M.S., Scheerer, P., Lipscomb, J.D., Weierstall, U., Kornberg, R.D., Spence, J.C., Pollack, L., Chapman, H.N., and Bajt, S. (2017). Double-flow focused liquid injector for efficient serial femtosecond crystallography. Scientific Reports 7, 44628; doi: 10.1038/srep44628
Oppermann, J., Fischer, P., Silapetere, A., Liepe, B., Rodriguez-Rozada, S., Flores-Uribe, J., Peter, E., Keidel, A., Vierock, J., Kaufmann, J., Broser, M., Luck, M., Bartl, F., Hildebrandt, P., Wiegert, J. S., Béjà, O., Hegemann, P., Wietek, J. (2019). MerMAIDs: a family of metagenomically discovered marine anion-conducting and intensely desensitizing channelrhodopsins. Nature Communications 10: 3315; doi: 10.1038/s41467-019-11322-6
Scheerer, P., Unger, E., and Tian, L. (2018). Protein structures guide the design of a much-needed tool for neuroscience. Nature 561, 312-313; doi: 10.1038/d41586-018-06670-0
Schmidt, A., Sauthof, L., Szczepek, M., Lopez, M., Velázquez, F., Qureshi, B. M., Michael, N., Buhrke, D., Stevens, T., Kwiatkowski, D., von Stetten, D., Mroginski, M. A., Krauß, N., Lamparter, T. , Hildebrandt, P., and Scheerer, P. (2018). Structural snapshot of a bacterial phytochrome in its functional intermediate state. Nature Communications 9, 4912; doi: 10.1038/s41467-018-07392-7
Takiden, A., Velazquez-Escobar, F., Dragelj, J., Woelke, A.L., Knapp E.-W., Piwowarski, P., Bartl, F., Hildebrandt, P., and Mroginski, M.A. (2017). Structural and Vibrational Characterization of the Chromophore Binding Site of Bacterial Phytochrome Agp1. Photochemistry and Photobiology 93, 713-723; doi: 10.1111/php.12737
Velázquez Escobar, F., Buhrke, D., Fernandez Lopez, M., Shenkutie, S.M., von Horsten., S, Essen, L.O., Hughes, J., and Hildebrandt, P. (2017). Structural communication between the chromophore-binding pocket and the N-terminal extension in plant phytochrome phyB. FEBS Letters 591, 1258-1265; doi: 10.1002/1873-3468.12642
Velázquez Escobar, F., Buhrke, D., Michael, N., Sauthof, L., Wilkening, S., Tavraz, N.N., Salewski, J., Frankenberg-Dinkel, N., Mroginski, M.A., Scheerer, P., Friedrich, T., Siebert, F., and Hildebrandt, P. (2017). Common Structural Elements in the Chromophore Binding Pocket of the Pfr State of Bathy Phytochromes. Photochemistry and Photobiology 93, 724-732; doi: 10.1111/php.12742
Velazquez Escobar, F., Kneip, C., Michael, N., Hildebrandt, T., Tavraz, N., Gärtner, W., Hughes, J., Friedrich, T., Scheerer, P., Mroginski, M.A., and Hildebrandt, P. (2020). The Lumi-R Intermediates of Prototypical Phytochromes. Journal of Physical Chemistry B 124, 4044-4055. doi: 10.1021/acs.jpcb.0c01059.
Velazquez Escobar, F., Lang, C., Takiden, A., Schneider, C., Balke, J., Hughes, J., Alexiev, U., Hildebrandt, P., and Mroginski, M.A. (2017). Protonation-Dependent Structural Heterogeneity in the Chromophore Binding Site of Cyanobacterial Phytochrome Cph1. Journal of Physical Chemistry B 121, 47-57; doi: 10.1021/acs.jpcb.6b09600
2013 - 2016
Bruun, S., Stoeppler, D., Keidel, A., Kuhlmann, U., Luck, M., Diehl, A., Geiger, M.-A., Woodmansee, D., Trauner, D., Hegemann, P., Oschkinat, H., Hildebrandt, P., and Stehfest, K. (2015). Light–Dark Adaptation of Channelrhodopsin Involves Photoconversion between the all-trans and 13-cis Retinal Isomers. Biochemistry 54, 5389-5400.
Buhrke, D., Velazquez Escobar, F., Sauthof, L., Wilkening, S., Herder, N., Tavraz, N.N., Willoweit, M., Keidel, A., Utesch, T., Mroginski, M.A., Schmitt, F.J., Hildebrandt, P., and Friedrich, T. (2016). The role of local and remote amino acid substitutions for optimizing fluorescence in bacteriophytochromes: A case study on iRFP. Scientific Reports 6, 28444.
Luck, M., Bruun, S., Keidel, A., Hegemann, P., and Hildebrandt, P. (2015). Photochemical chromophore isomerization in histidine kinase rhodopsin HKR1. FEBS Letters 589, 1067-1071.
Nagano S., Scheerer P., Zubow K., Michael N., Inomata K., Lamparter T., Krauß N. (2016). The Crystal Structures of the N-terminal Photosensory Core Module of Agrobacterium Phytochrome Agp1 as Parallel and Anti-parallel Dimers. Journal of Biological Chemistry 291, 20674-20691.
Otero, L.O., Klinke, S., Rinaldi, J., Velazquez Escobar, F., Mroginski, M.A., Fernandez Lopez, M., Malamud, F., Vojnov, A., Hildebrandt, P., Goldbaum, F.A., and Bonomi, H.R. (2016). Structure of the full-length bacteriophytochrome from the plant pathogen Xanthomonas campestris provides clues to its long-range signaling mechanism. Journal of Molecular Biology 428, 3702-3720.
Salewski, J., Escobar, F.V., Kaminski, S., von Stetten, D., Keidel, A., Rippers, Y., Michael, N., Scheerer, P., Piwowarski, P., Bartl, F., Frankenberg-Dinkel, N., Ringsdorf, S., Gaertner, W., Lamparter, T., Mroginski, M.A., and Hildebrandt, P. (2013). Structure of the Biliverdin Cofactor in the Pfr State of Bathy and Prototypical Phytochromes. Journal of Biological Chemistry 288, 16800-16814.
Song, C., Velazquez Escobar, F., Xu, X.-L., Narikawa, R., Ikeuchi, M., Siebert, F., Gärtner, W., Matysik, J., and Hildebrandt, P. (2015). A Red/Green Cyanobacteriochrome Sustains Its Color Despite a Change in the Bilin Chromophore’s Protonation State. Biochemistry 54, 5839-5848.
Velazquez Escobar, F., Hildebrandt, T., Utesch, T., Schmitt, F.J., Seuffert, I., Michael, N., Schulz, C., Mroginski, M.A., Friedrich, T., and Hildebrandt, P. (2014). Structural Parameters Controlling the Fluorescence Properties of Phytochromes. Biochemistry 53, 20-29.
Velazquez Escobar, F., Piwowarski, P., Salewski, J., Michael, N., Fernandez Lopez, M., Rupp, A., Muhammad Qureshi, B., Scheerer, P., Bartl, F., Frankenberg-Dinkel, N., Siebert, F., Mroginski, M.A., and Hildebrandt, P. (2015). A protonation-coupled feedback mechanism controls the signalling process in bathy phytochromes. Nature Chemistry 7, 423-430. [Link to news section.]
Velazquez Escobar, F., Utesch, T., Narikawa, R., Ikeuchi, M., Mroginski, M.A., Gaertner, W., and Hildebrandt, P. (2013). Photoconversion Mechanism of the Second GAF Domain of Cyanobacteriochrome AnPixJ and the Cofactor Structure of Its Green-Absorbing State. Biochemistry 52, 4871-4880.
Velazquez Escobar, F., von Stetten, D., Günther-Lütkens, M., Keidel, A., Michael, N., Lamparter, T., Essen, L.-O., Hughes, J., Gärtner, W., Yang, Y., Heyne, K., Mroginski, M.A., and Hildebrandt, P. (2015). Conformational heterogeneity of the Pfr chromophore in plant and cyanobacterial phytochromes. Frontiers in Molecular Biosciences 2, 37.
Zienicke, B., Molina, I., Glenz, R., Singer, P., Ehmer, D., Escobar, F.V., Hildebrandt, P., Diller, R., and Lamparter, T. (2013). Unusual spectral properties of bacteriophytochrome Agp2 result from a deprotonation of the chromophore in the red-absorbing form Pr. Journal of Biological Chemistry 288, 31738-31751.